Sacrificial Anodes
Sacrificial anodes for boats are a relatively cheap way of protecting the key parts of your boat from corrosion due to the effects of salt-water and electrical currents. Anodes are actually the one part (save for anti-fouling) that you want to degrade during the sailing season. Seeing your sacrificial anode being gradually eaten away (as long as not too fast) gives you confidence that it is doing its job of protecting the other metallic parts of your boat around and below the water-line.
Quick Links To Common Questions / Answers:
- What are the most suitable metals for sacrificial anodes
- How many anodes do I need
- Where is the best place to fit an anode or anodes
- How often should I replace an anode
- How can I tell if my anode is working
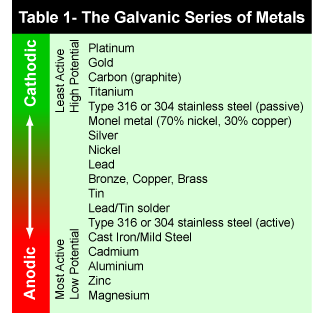
There are two types of corrosion that we need to be wary of as boat owners, Galvanic Corrosion and Electrolytic Corrosion. Galvanic corrosion is caused when parts made from different metals are joined or connected to each other while in water. When dis-similar metals are put into water, a voltage is produced, effectively creating a battery causing one of the components to corrode. Which part will corrode depends on their relative position in the galvanic series. The part of the boat lower down in the series will form the cathode, and the one higher up in the series will be the anode. The anode is the part which will be corroded.
For example when a brass propeller connected to a stainless steel prop-shaft, the brass propeller is the anode and the prop-shaft is the cathode. This means that the brass propeller would be the component to suffer corrosion. Referring to table 1 we can see that Brass is the more ‘Anodic’ of the two materials.
Table 1: The Galvanic Series of Metals
The effect of galvanic corrosion is exaggerated in salt-water compared to fresh-water but in both cases anodes will have a beneficial effect, and are a relatively low-cost item compared to the cost of replacing parts of your boat.
Electrolytic Corrosion
Electrolytic corrosion occurs when stray currents from your boat electrical system or from a shore power system find their way through your hull or hull fitting into the water. The currents in this situation can be much greater and cause more damage than those from galvanic corrosion.
Perhaps not surprisingly the three commonly used materials for sacrificial anodes are the three at the bottom of the table, namely Aluminum, Zinc and Magnesium. Selecting which material is best for your sacricial anode depends on the type of water that the boat is mainly used in.
Aluminium anodes are suited to brackish water or salt water. Aluminium anodes are not suited to fresh water as prolonged exposure to fresh water can lead to the build up of an oxide layer which prevents the anode from working effectively.
Zinc anodes are suited to salt water, but not recommended for fresh water as prolonged exposure to fresh water (and to some extent brackish water) can lead to the build up of an oxide layer which prevents the anode from working once the boat is back in salt-water.
Magnesium anodes are suited to fresh water. Magnesium is not suited to salt-water as they are too active in this situation and will corrode too quickly.
How Many / What Size Of Anodes Do I need
This depends on the type of water and the type and size of boat and usage. A handy tool for guiding you in this respect can be found here:
https://mgduff.co.uk/find-your-anode/hull-anode-search
Where is the best place to fit an anode or anodes
Anodes should be fitted to each propeller shaft assembly, and often it is advisable to fit additional anodes for protecting trim tabs and rudders as well as the hull / keel areas. Here is an example of an sacrificial anode (or range of anodes) which can be purchased from boatfittings.co.uk for protecting propellers and propeller shaft assemblies:
How often should I replace anodes
When an anode is approaching 50% of its original size it should be replaced. If in doubt we would recommend erring on the side of caution. We would recommend inspecting anodes at least every season.
If you would like any further advice please do not hesitate to get in contact with us here at Boatfittings by phone or email.
If you found this guide useful or interesting you might like to sign up to our newsletter, so that we can let you know when our next guide has been published.





